(Updated August 2021)
When developing a medical injection molding process with repeatability as the cornerstone, does it really matter how you get there? Every medical injection molding supplier has their own internal validation process, but when you pull back the curtain and dig into the details as to how it was developed there might be some issues that arise.
The development of a medical injection molding process really shouldn’t be that much different than other industries, but there are some key components that are specific to medical injection molding. After 50+ years of serving the medical market, Crescent Industries has created the most robust processes for repeatability.
The History
Robotics, scientific injection molding principles and fully automated cells weren’t an option back in the 1970’s when Crescent Industries first started serving the medical markets. The technology was different, but the goal of producing repeatable quality medical injection molded parts was always the most important value that Crescent Industries held.
Over the 50+ year journey serving the medical markets, Crescent Industries has continued to stay at the forefront of technology and investing back into the company. These investments were focused around common questions during management meetings:
- How can we make a more robust process?
- What technology is available to assist?
- How can we exceed industry standards?
This commitment has brought Crescent Industries to a level that is unmatched at any other medical injection molding supplier. By utilizing the latest technology in robotics and scientific molding and continuously improving internal validation processes, Crescent has earned it’s mark as the go to medical injection molding supplier. The mindset of raising the medical injection molding theoretical “bar” has pushed Crescent into a class on their own, often having their customers citing them as level they hold their other suppliers to.
Procedures and Processes
As the ISO 13485 standard has continued to evolve, this is only a singular aspect of a medical injection molding process. The standard sets certain processes, documentation and criteria that must be met, but it does not determine how a company develops a molding process or validation of that process. While Crescent holds the latest ISO 13485:2016 certification and is FDA registered, the internal standards and SOP’s dictate the process development.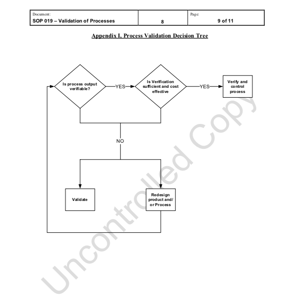
All process validation must be determined using a “Validation Decision Tree” and then documented in the “Validation Master Plan” which is located in IQMS (best ERP system of plastic companies). Through the validation process, there are documented internal forms that states the protocol, checklist and report as well as IQ, OQ, PQ standards that must be followed.
IQ (Installation Qualification)
- Equipment design features
- Utility Requirements
- Calibration, Preventative maintenance, cleaning schedules
- Safety features
- Supplier documentation
- Software & Spare parts list
- Environmental conditions (cleanroom, temperature, humidity, cleanliness, etc.)
- Ancillary equipment requirements (includes mold)
OQ (Operational Qualification)
- Process control limits (time, temperature, pressure, speed, etc.)
- Software parameters
- Raw material specifications
- Process operating procedures
- Material handling requirements
- Process change control
- Training
- Short term capability and stability
- Failure modes, action level, worst case conditions as determined in process FMEA and process development
- Measurement methods; gauge reliability and reproducibility.
PQ (Performance Qualification)
- Nominal process parameters
- Adherence to requirements
- Process Capability
- Process Stability
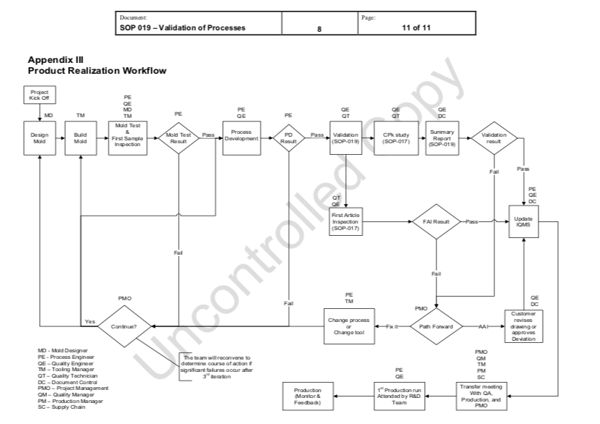
Through a comprehensive “Product Realization Workflow”, Crescent Industries is able to create a robust process from the initial kickoff of a project through full production approval with each step identifying which departments and internal team members will be involved.
Scientific Injection Molding Importance
Though having a robust internal process to develop and validate a medical injection molding product is the foundation, utilizing scientific injection molding principles creates the most robust molding process.
Scientific injection molding is a disciplined approach to using data backed decisions to setup consistent and repeatable molding processes. By creating these extremely robust molding processes, predictive quality and consistent repeatability is the outcome.
This is accomplished through 4 phases:
- Material Selection
- Part Design
- Tooling Design
- Process Development
The benefits behind scientific over traditional molding at the highest level are significant for shot-to-shot consistency, predictable quality, lower scrap rates and a more robust part.
The main two areas of equipment used in scientific injection molding by RJG is the eDART® System and the in-mold sensors. These two pieces are used to communicate together along with the machine to give the injection molder a view into what’s happening in the process that they aren’t aware of.
In a traditional molding environment, processes are based on machine input. The process engineer set’s up the machine based on the inputs they enter; where it’s position, time, temperature, pressure, etc. The injection molding machine is supposed to execute on those inputs, however without secondary equipment you don’t really know whether or not those inputs where actually achieved.
The scientific molding approach instead focuses on outputs. Regardless of what your input is, by focusing on the output you can setup a more robust process based on actual data from the cycle. To see what the output is, you need the eDART® System and the in-mold sensors to communicated with the machine and tell you what’s happening in the mold during the cycle.
To learn more about scientific injection molding and how Crescent Industries utilizes it for medical injection molding, check out the White Paper below:
Scientific Injection Molding Processes and Benefits
What’s Your Standard?
While there are a lot of medical injection molding suppliers that are chomping at the bit to take on a new project, raising the standard of supplier selection is necessary to partner with the right company. Valid certification in ISO 13485:2016 shouldn’t be the only criteria needed to past the initial test, potential suppliers should be providing documentation and real-life examples of how the molding process development and validation are achieved.
Simply making a “good part” isn’t enough to compete with companies like Crescent Industries, showing consistent repeatability and utilizing the latest technology in automation and scientific injection molding should be the new standard.
If you’re interested in learning how our Crescent Industries can utilize the robust process development and validation process on your medical injection molding project, please click here to send us over an RFQ!


